What Cause Cytokinesis In Plant Cells But Not In Animal Cells?
The cell cycle culminates in the division of the cytoplasm by cytokinesis. In a typical cell, cytokinesis accompanies every mitosis, although some cells, such every bit Drosophila embryos (discussed later) and vertebrate osteoclasts (discussed in Chapter 22), undergo mitosis without cytokinesis and become multinucleate. Cytokinesis begins in anaphase and ends in telophase, reaching completion equally the next interphase begins.
The first visible change of cytokinesis in an animal cell is the sudden appearance of a pucker, or cleavage furrow, on the cell surface. The furrow chop-chop deepens and spreads around the cell until it completely divides the cell in two. In animal cells and many unicellular eucaryotes, the construction that accomplishes cytokinesis is the contractile ring—a dynamic assembly equanimous of actin filaments, myosin II filaments, and many structural and regulatory proteins. The ring assembles just below the plasma membrane and contracts to constrict the cell into two (see Figure 18-iv). At the same time, new membrane is inserted into the plasma membrane adjacent to the contractile band by the fusion of intracellular vesicles. This addition of membrane is required to compensate for the increase in surface surface area that accompanies cytoplasmic division. Thus, cytokinesis can exist considered to occur in four stages—initiation, wrinkle, membrane insertion, and completion.
The central problem for a cell undergoing cytokinesis is to ensure that it occurs at the right time and in the right place. Cytokinesis must not occur too early in Grand-stage, or it will disrupt the path of the separating chromosomes. It must besides occur at the right place to separate the ii segregating sets of chromosomes properly so that each daughter cell receives a complete set.
The Microtubules of the Mitotic Spindle Determine the Plane of Animal Cell Division
The mitotic spindle in animal cells not simply separates the girl chromosomes, information technology too specifies the location of the contractile ring, and thereby the plane of prison cell sectionalization. The contractile ring invariably forms in the plane of the metaphase plate, at right angles to the long centrality of the mitotic spindle, thereby ensuring that partitioning occurs betwixt the two sets of separated chromosomes. The part of the spindle that specifies the division aeroplane varies depending on the jail cell blazon: in some cells, it is the astral microtubules; in others, it is the overlapping antiparallel microtubules in the central spindle.
The human relationship between the spindle microtubules and the placement of the contractile band has been studied past manipulating fertilized eggs of marine invertebrates. Afterward fertilization, these embryos undergo a series of rapid cleavage divisions, without intervening periods of growth. In this style, the original egg is progressively divided up into smaller and smaller cells. During cytokinesis, the cleavage furrow appears suddenly on the surface of the cell and deepens rapidly (Figure xviii-thirty). Considering the cytoplasm is clear, the spindle can be observed in real time through a microscope. If the spindle is tugged into a new position with a fine glass needle in early anaphase, the incipient cleavage furrow disappears, and a new one develops in accord with the new spindle site.

Figure 18-30
Cleavage in a fertilized frog egg. In these scanning electron micrographs, the cleavage furrow is especially obvious and well defined, as the prison cell is unusually large. The furrowing of the cell membrane is caused by the activity of the contractile band (more...)
How does the mitotic spindle control the plane of division? Ingenious experiments in large embryonic cells demonstrate that a cleavage furrow forms midway between the asters originating from the two centrosomes, fifty-fifty when the two centrosomes are not connected to each other by a mitotic spindle (Figure xviii-31). Thus, in these cells, the microtubule asters—non the chromosomes or other parts of the spindle—betoken to the cell cortex to specify where the contractile band should assemble. In other cells, the central spindle, rather than the astral microtubules, is manifestly responsible for this specification. In either example, it has been speculated that the overlapping microtubules may provide tracks for motor proteins to evangelize contractile band regulators, and perhaps new membrane, to the appropriate region of the dividing prison cell. But, in fact, the molecular machinery by which the spindle positions the cleavage furrow remains a mystery.

Figure 18-31
An experiment demonstrating the influence of the position of microtubule asters on the subsequent airplane of cleavage in a large egg cell. If the mitotic spindle is mechanically pushed to one side of the cell with a glass bead, the membrane furrowing is (more...)
In some cells, the site of band associates is chosen before mitosis, according to a landmark placed in the cortex during a previous jail cell bicycle. In budding yeasts, for example, a ring of proteins chosen septins assembles earlier mitosis, adjacent to a bud scar left on the cell surface as the mother and girl cells separated in the previous segmentation. The septins are thought to course a scaffold onto which other components of the contractile ring, including myosin II, gather. As we discuss afterward, in plant cells, an organized band of microtubules and actin filaments assembles just before mitosis and marks the site where the cell wall volition get together and divide the cell in two.
Some Cells Reposition Their Spindle to Split up Asymmetrically
Most cells divide symmetrically. In most animal cells, for example, the contractile ring forms around the equator of the parent prison cell, so that the two daughter cells produced are of equal size and have similar properties. This symmetry results from the placement of the mitotic spindle, which in most cases tends to center itself in the cytoplasm. The centering procedure depends both on astral microtubules and on motor proteins that either push or pull on the astral microtubules to center the spindle.
There are many instances in evolution, nonetheless, when cells split up asymmetrically to produce two cells that differ in size, in the cytoplasmic contents they inherit, or in both. Unremarkably, the two daughter cells are destined to develop forth different pathways. To create daughter cells with unlike fates, the mother prison cell must first segregate some components (called fate determinants) to i side of the cell so position the aeroplane of division so that the appropriate daughter cell inherits these components (Figure 18-32). To position the plane of partitioning asymmetrically, the spindle has to be moved in a controlled style within the dividing cell. Information technology seems likely that such spindle movements are directed by changes in local regions of the cell cortex and that motor proteins localiazed at that place pull one of the spindle poles, via its astral microtubules, to the appropriate region (Figure 18-33). Some of the proteins required for such asymmetrical divisions have been identified through genetic analyses in C. elegans and Drosophila (discussed in Chapter 21), and some of these seem to take a like role in vertebrates.

Effigy 18-32
An asymmetric cell partitioning segregating cytoplasmic components to only 1 girl cell. These light micrographs illustrate the controlled disproportionate segregation of specific cytoplasmic components to ane girl cell during the offset division of a fertilized (more than...)

Figure 18-33
Spindle rotation. (A) A possible machinery underlying the controlled rotation of a mitotic spindle. The red bar represents a specialized region of cell cortex toward which one spindle pole is pulled by its astral microtubules. (B) Fluorescence micrographs (more...)
Disproportionate division is peculiarly important in plant cells. Every bit these cells cannot motility later division, the selection of segmentation planes is crucial for controlling tissue morphology. Nosotros discuss later how the airplane of division is determined in these cells.
Actin and Myosin II in the Contractile Ring Generate the Force for Cytokinesis
As the astral microtubules in anaphase go longer lived and less dynamic in response to the loss of M-Cdk activity, the contractile band begins to gather below the plasma membrane. Much of the preparation for cytokinesis, withal, happens earlier in mitosis, earlier the division of the cytoplasm really begins. In interphase cells, actin and myosin filaments are assembled into a cortical network and, in some cells, as well into big cytoplasmic bundles chosen stress fibers (discussed in Chapter 16). Equally cells enter mitosis, these arrays detach; much of the actin is reorganized, and myosin Ii filaments are released. Every bit the chromatids separate in anaphase, myosin II begins to accrue in the rapidly assembling contractile band (Effigy 18-34).

Effigy 18-34
The contractile ring. (A) A drawing of the cleavage furrow in a dividing cell. (B) An electron micrograph of the ingrowing edge of a cleavage furrow of a dividing beast cell. (C) Fluorescence micrographs of a dividing slime mold amoeba stained for actin (more...)
In many cells, cytokinesis requires the activation of i or more members of the polo-like family of protein kinases. These kinases regulate the assembly of both the mitotic spindle and the contractile band and are therefore thought to help coordinate mitosis and cytokinesis, just it is uncertain how they do so. The fully assembled contractile ring contains many proteins in addition to actin and myosin Ii. The overlapping arrays of actin filaments and bipolar myosin 2 filaments, nevertheless, generate the strength that divides the cytoplasm in two. They are thought to contract by a mechanism that is biochemically similar to that used by smooth muscle cells; in both cases, for example, the contraction begins when Catwo+-calmodulin activates myosin light-chain kinase to phosphorylate myosin Ii. Once contraction has been stimulated, the ring develops a force large enough to bend a fine drinking glass needle that is inserted in the path of the constricting band.
How the contractile ring constricts is still a mystery. It seems non to operate past a uncomplicated "purse-string" mechanism, with actin and myosin II filaments sliding past each other as in skeletal muscle (see Effigy 16-71). As the ring constricts, the ring maintains the same thickness in cantankerous-department, suggesting that its total volume and the number of filaments it contains subtract steadily. Moreover, dissimilar in muscle, the actin filaments in the ring are highly dynamic, and their arrangement changes extensively during cytokinesis.
In addition to specifying the site of contractile ring assembly in early anaphase, in many cells, microtubules also work continuously during anaphase and telophase to stabilize the advancing cleavage furrow. Drugs that depolymerize microtubules, for example, cause the actin filaments in the contractile band to become less organized. Moreover, if a needle is used to tear microtubules away from the prison cell cortex, the contractile band disassembles and the cleavage furrow regresses. It is not known how the microtubules stabilize the ring, although it has been shown that growing microtubules can activate some members of the Rho family of small GTPases, which in plough stimulate actin polymerization (discussed in Chapter 16). One fellow member of this family, Rho A, is required for cytokinesis.
The contractile band is finally dispensed with birthday when cleavage ends, equally the plasma membrane of the cleavage furrow narrows to class the midbody. The midbody persists equally a tether between the ii daughter cells and contains the remains of the central spindle, which now consists of the two sets of antiparallel overlap microtubules packed tightly together within a dumbo matrix material (Figure 18-35). Remarkably, in some cells, before cytokinesis has been completed, the female parent centriole from one or both daughter cells separates from its girl centriole (meet Figure 18-5c) and migrates into the midbody, where it lingers for minutes, before returning to its daughter prison cell. Only then do the two daughter cells separate to complete cytokinesis. What the centriole might practice in the midbody to trigger the last steps of cytokinesis is not known. Subsequently the daughter cells carve up completely, some of the components of the residual midbody often remain on the within of the plasma membrane of each prison cell, where they may serve as a mark on the cortex that helps to orient the spindle in the subsequent cell sectionalisation.

Effigy 18-35
The midbody. (A) A scanning electron micrograph of an beast cell in civilization in the process of dividing; the midbody still joins the two daughter cells. (B) A conventional electron micrograph of the midbody of a dividing animal jail cell. Cleavage is almost (more...)
Membrane-enclosed Organelles Must Exist Distributed to Daughter Cells During Cytokinesis
The process of mitosis ensures that each daughter prison cell receives a total complement of chromosomes. But when a eucaryotic prison cell divides, each daughter cell must as well inherit all of the other essential jail cell components, including the membrane-enclosed organelles. As discussed in Chapter 12, organelles like mitochondria and chloroplasts cannot assemble spontaneously from their private components; they tin arise only from the growth and division of the preexisting organelles. Similarly, cells cannot make a new endoplasmic reticulum (ER) unless some office of it is already present.
How, then, are the diverse membrane-enclosed organelles segregated when a cell divides? Organelles such every bit mitochondria and chloroplasts are usually present in big enough numbers to be safely inherited if, on average, their numbers roughly double one time each bicycle. The ER in interphase cells is continuous with the nuclear membrane and is organized by the microtubule cytoskeleton. Upon entry into Grand stage, the reorganization of the microtubules releases the ER, which fragments every bit the nuclear envelope breaks downwards. The Golgi apparatus probably fragments as well, although in some cells it seems to redistribute transiently into the ER, but to re-emerge at telophase. Some of the organelle fragments associate with the spindle microtubules via motor proteins, thereby hitching a ride into the daughter cells every bit the spindle elongates in anaphase.
Mitosis Tin can Occur Without Cytokinesis
Although nuclear division is ordinarily followed by cytoplasmic sectionalisation, in that location are exceptions. Some cells undergo multiple rounds of nuclear partitioning without intervening cytoplasmic division. In the early Drosophila embryo, for example, the beginning xiii rounds of nuclear partition occur without cytoplasmic sectionalization, resulting in the formation of a single large cell containing 6000 nuclei, arranged in a monolayer near the surface (Figure eighteen-36). This system profoundly speeds upwards early development, every bit the cells practice not have to have the time to go through all the steps of cytokinesis for each segmentation. After these rapid nuclear divisions, cells are created around each nucleus in 1 circular of coordinated cytokinesis called cellularization. Contractile rings class at the cell surface, and the plasma membrane extends inward and pinches off to enclose each nucleus.

Effigy eighteen-36
Mitosis without cytokinesis in the Drosophila embryo. (A) The get-go xiii nuclear divisions occur synchronously and without cytoplasmic partitioning to create a large syncytium. Most of the nuclei then migrate to the cortex, and the plasma membrane extends (more...)
Nuclear division without cytokinesis also occurs in some types of mammalian cells. Osteoclasts, trophoblasts, and some hepatocytes and heart muscle cells, for example, become multinucleated in this way.
The Phragmoplast Guides Cytokinesis in College Plants
Nearly higher-institute cells are enclosed past a semirigid cell wall, and their mechanism of cytokinesis is different from that just described for animal cells. Rather than a contractile ring dividing the cytoplasm from the outside in, the cytoplasm of the plant jail cell is partitioned from the inside out by the structure of a new cell wall, called the cell plate, between the two daughter nuclei (Effigy xviii-37). The orientation of the cell plate determines the positions of the two daughter cells relative to neighboring cells. It follows that altering the planes of cell sectionalisation, together with enlargement of the cells by expansion or growth, leads to dissimilar jail cell and tissue shapes that help decide the form of the found.

Effigy 18-37
Cytokinesis in a constitute cell in telophase. In this light micrograph, the early cell plate (between the two arrowheads) is forming in a plane perpendicular to the plane of the page. The microtubules of the spindle are stained with aureate-labeled antibodies (more...)
The mitotic spindle by itself is not sufficient to determine the exact position and orientation of the prison cell plate. The first visible sign that a college-plant cell has become committed to split in a particular plane is seen in G2, when the cortical array of microtubules disappears in preparation for mitosis. At this time, a circumferential band of microtubules and actin filaments forms a band effectually the unabridged jail cell only beneath the plasma membrane. Because this cytoskeletal array appears before prophase begins, it is called the preprophase band. The ring becomes thinner every bit the cell progresses to prophase, and information technology disappears completely before metaphase is reached. All the same, the division plane has somehow been established: when the new cell plate forms later during cytokinesis, information technology grows outward to fuse with the parental wall precisely at the zone formerly occupied by the preprophase band. Even if the cell contents are displaced by centrifugation afterward the preprophase band has disappeared, the growing cell plate tends to find its way back to the aeroplane defined by the former preprophase band.
The assembly of the cell plate begins in late anaphase and is guided past a structure called the phragmoplast, which contains the remaining overlap microtubules of the mitotic spindle that interdigitate at their growing plus ends. This region of overlap is similar in structure to the fundamental spindle in brute cells in late anaphase. Small vesicles, largely derived from the Golgi apparatus and filled with polysaccharide and glycoproteins required for the synthesis of the new cell-wall matrix, are transported forth the microtubules to the equator of the phragmoplast, apparently by the action of microtubule-dependent motor proteins. Here, the vesicles fuse to form a disclike, membrane-enclosed structure called the early cell plate (come across Figure 18-9G). The plate expands outward by further vesicle fusion until information technology reaches the plasma membrane and the original cell wall and divides the cell in 2. Later, cellulose microfibrils are laid downwards within the matrix of the cell plate to complete the structure of the new jail cell wall (Figure eighteen-38).

Figure eighteen-38
The special features of cytokinesis in a higher plant prison cell. The division plane is established before M phase past a band of microtubules and actin filaments (the preprophase ring) at the jail cell cortex. At the start of telophase, later on the chromosomes (more than...)
The Elaborate M Phase of Higher Organisms Evolved Gradually from Procaryotic Fission Mechanisms
Procaryotic cells split by a procedure called binary fission. The single, circular DNA molecule replicates and division occurs by the invagination of the plasma membrane and the laying down of new cell wall between the two chromosomes to produce 2 carve up girl cells. In East. coli, before the chromosome replicates, the single origin of replication (oriC) is located at one pole of the rod-shaped bacterium. As soon equally oriC is replicated, one copy of the sequence is immediately translocated to the opposite pole of the prison cell, after which the residual of the chromosome is replicated. Similar the ii spindle-pole asters in an creature cell, the bacterial daughter chromosomes at the prison cell poles somehow decide the location of the aeroplane of cell division, ensuring that fission takes identify at the cell equator, so that each daughter jail cell inherits one chromosome (Figure 18-39). Although a number of genes and proteins involved have been identified, the mechanisms responsible for the active translocation of oriC and the inhibition of fission everywhere but at the equator remain unknown.
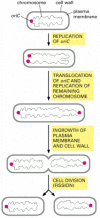
Effigy xviii-39
Cell partitioning in the bacterium E. coli. The single, round chromosome contains an origin of replication called oriC. Before partition, the chromosome is polarized, so that oriC is at one pole of the bacterium. As soon as the oriC sequence is copied, (more...)
Binary fission in procaryotes depends on filaments fabricated of the FtsZ protein. FtsZ is a cytoskeletal GTPase that is structurally related to tubulin and assembles into a ring at the equator of the cell (Figure 18-40A, and see Figure 16-17). The FtsZ filaments are essential for the recruitment of all the other cell sectionalization proteins to the division site. Together, these proteins guide the inwards growth of the cell wall and membrane, leading to the formation of a septum that divides the cell into two. Bacteria in which the ftsZ gene is inactivated by mutation cannot divide. A FtsZ-based mechanism is also used in the sectionalisation of chloroplasts in plant cells (Figure 18-40B) and mitochondria in protists. In fungi and animal cells, some other self-assembling GTPase called dynamin (discussed in Affiliate 13) has apparently taken over the role of FtsZ in mitochondrial division.
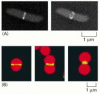
Figure 18-forty
The FtsZ protein. (A) Fluorescence micrographs showing the location of the FtsZ poly peptide during binary fission in E. coli. The protein assembles into a ring at the centre of the cell, where it helps orchestrate cell division. The bacteria here have been (more...)
With the evolution of the eucaryotes, the genome increased in complexity, and the chromosomes increased in both number and size. For these organisms, a more elaborate mechanism for dividing the chromosomes between girl cells was apparently required. Clearly, the mitotic apparatus could not have evolved all at once. In many primitive eucaryotes, such as the dinoflagellate Cryphthecodinium cohnii, mitosis depends on a membrane-attachment mechanism, in which the chromosomes take to demark to the inner nuclear membrane for segregation. The intermediate status of this large, unmarried-celled alga is reflected in the composition of its chromosomes, which, similar those of procaryotes, have relatively piffling associated protein. The nuclear membrane in C. cohnii remains intact throughout mitosis, and the spindle microtubules remain entirely exterior the nucleus. Where these spindle microtubules press on the outside of the nuclear envelope, the envelope becomes indented in a series of parallel channels (Figure 18-41). The chromosomes get attached to the inner membrane of the nuclear envelope contrary these channels, and chromosome segregation occurs on the within of this channeled nuclear membrane. Thus, the extranuclear "spindle" is used to order the nuclear membrane and thereby ascertain the plane of division. Kinetochores in this species seem to exist integrated into the nuclear membrane and may therefore have evolved from some membrane component.
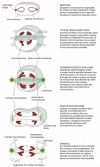
Figure 18-41
The use of dissimilar chromosome separation mechanisms by unlike organisms. Some of these may have been intermediate stages in the development of the mitotic spindle of higher organisms. For all examples except bacteria, just the fundamental nuclear region (more...)
Eucaryotic tubulin and procaryotic FtsZ clearly have a common evolutionary history. But, microtubules are important for chromosome segregation in even the most primitive eucaryotes, where they are also present in flagellar axonemes (discussed in Affiliate sixteen). Whether the flagellum or the spindle evolved showtime is unclear.
A somewhat more advanced, although still extranuclear, spindle is seen in hypermastigotes, in which the nuclear envelope again remains intact throughout mitosis. These large protozoa from the guts of insects provide a particularly clear illustration of the independence of spindle elongation and the chromosome movements that split up the chromatids. The sister kinetochores get separated by the growth of the nuclear membrane (to which they are attached) earlier condign fastened to the spindle. But when the kinetochores are near the poles of the spindle do they acquire the kinetochore microtubules needed to attach them to the spindle. Because the spindle microtubules remain separated from the chromosomes by the nuclear envelope, the kinetochore microtubules, which are formed exterior the nucleus, must somehow attach to the chromosomes through the nuclear membranes. Later on this attachment has occurred, the kinetochores are drawn poleward in a conventional way (see Figure 18-41).
Organisms that class spindles within an intact nucleus may represent a further stage in the development of mitotic mechanisms. In both yeasts and diatoms, the spindle is attached to chromosomes past their kinetochores, and the chromosomes are segregated in a mode loosely similar to that described for animal cells—except that the entire process mostly occurs within the confines of the nuclear envelope (see Figure 18-41). It is idea that the "open" mitosis of higher organisms and the "closed" mitosis of yeasts and diatoms evolved separately from a common ancestor resembling the modernistic hypermastigote spindle. At present, there is no convincing explanation for why higher plants and animals accept evolved a mitotic apparatus that requires the controlled and reversible dissolution of the nuclear envelope.
Summary
Cell division ends as the cytoplasm divides into two past the process of cytokinesis. Except for plants, cytokinesis in eucaryotic cells is mediated by a contractile ring, which is composed of actin and myosin filaments and a diversity of other proteins. By an unknown mechanism, the mitotic spindle determines when and where the contractile ring assembles and, thereby, when and where the cell divides. Nearly cells divide symmetrically to produce two cells of the aforementioned content and size. Some cells, notwithstanding, specifically position their spindle to divide asymmetrically, producing 2 daughter cells that differ in size, content, or both. Cytokinesis occurs by a special mechanism in higher-plant cells—in which the cytoplasm is partitioned by the structure of a new cell wall, the cell plate, inside the cell. The position of the cell plate is determined past the position of a preprophase band of microtubules and actin filaments. The organization of mitosis in fungi and some protozoa differs from that in animals and plants, suggesting how the complex process of eucaryotic prison cell division may have evolved.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26831/
Posted by: duryeapecter.blogspot.com

0 Response to "What Cause Cytokinesis In Plant Cells But Not In Animal Cells?"
Post a Comment